- Research
- Publication
Published:
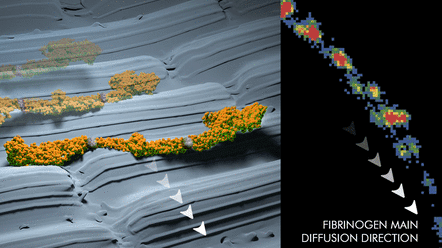
Surface nanostructures are increasingly more employed for controlled protein assembly on functional nanodevices, in nanobiotechnology, and in nanobiomaterials. However, the mechanism and dynamics of how nanostructures induce order in the adsorbed protein assemblies are still enigmatic. Here, we use single-molecule mapping by accumulated probe trajectories and complementary atomic force microscopy to shed light on the dynamic of in situ assembly of human plasma fibrinogen (HPF) adsorbed on nanostructured polybutene-1 (PB-1) and nanostructured polyethylene (PE) surfaces. We found a distinct lateral heterogeneity of HPF-polymer nanostructure interface (surface occupancy, residence time, and diffusion coefficient) that allow identifying the interplay between protein topographical nanoconfinement, protein diffusion mechanism, and ordered protein self-assembly. The protein diffusion analysis revealed high-diffusion polarization without correlation to the anisotropic friction characteristic of the polymer surfaces. This suggests that HPF molecules confined on the nanosized PB-1 needle crystals and PE shish-kebab crystals, respectively, undergo partial detachment and diffuse via a Sansetsukon-like nanocrawling mechanism. This mechanism is based on the intrinsic flexibility of HPF in the coiled-coil regions. We conclude that nanostructured surfaces that encourage this characteristic surface mobility are more likely to lead to the formation of ordered protein assemblies and may be useful for advanced nanobiomaterials.
Publication
Xiaoyuan Zhang, Izabela Firkowska-Boden, Matthias M. L. Arras, Mark J. Kastantin, Christian Helbing, Alper Özogul, Enrico Gnecco, Daniel K. Schwartz, Klaus D. Jandt: "Nanoconfinement and Sansetsukon-like Nanocrawling Govern Fibrinogen Dynamics and Self-Assembly on Nanostructured Polymeric Surfaces", Langmuir 34 (2018) 14309–14316, DOI: 10.1021/acs.langmuir.8b02917External link